Reports
Blueprints for Indication-Specific Pricing
Several approaches that rewire existing reimbursement conventions as alternatives to facilitate ISP are proposed.
Download Report
Originally published
on 05/19/2020
Drug Pricing Lab
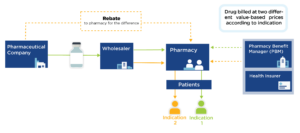
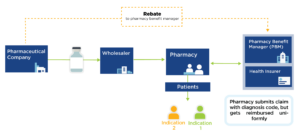
Interest in value-based pricing has increased in recent years as an analytic alternative to current pricing methods that are distorted by financial incentives across the industry and supply chain. Developing such a pricing model, however, is very complex, in part because many drugs can have different benefit profiles in different conditions.
Indication-specific pricing (ISP) is being tested by ExpressScripts and CVS Caremark to manage certain drugs according to price relative to indication-specific value. While promising, ISP requires mechanisms to reimburse by indication, which are currently hampered by the complexities of drug purchasing and delivery.
Share