Frequently Asked Questions
What is value-based pricing?
Value-based pricing is when the price of a drug is based on its measured benefits, for instance, in clinical trials leading to its approval. Methods used to determine value-based prices are transparent, reproducible and data driven.
What is outcomes-based contracting?
Outcomes-based contracting refers to arrangements between manufacturers and payers, in which the manufacturer is obligated to issue a refund or rebate to the payer that is linked to how well the therapy performs in a real-world population. This refund or rebate is off of a list price that the manufacturer sets. The methods manufacturers use to generate list prices are typically opaque, inconsistent, and driven more by market factors than clinical data. These methods are often referred to, by manufacturers as “pricing to what the market will bear”.
What is the difference between value-based pricing and outcomes-based contracting?
We can look to Amgen’s recent outcomes-based agreement with Harvard Pilgrim for its cholesterol medication, Repatha, to illustrate the difference.
Amgen has agreed to refund Harvard Pilgrim the cost of medication for patients who have a heart attack or stroke, an estimated 3.5% of individuals on the drug. This equates to a reduction in annual list price from $14,100 to $13,620. In contrast, the Institute for Clinical and Economic Review last reported that a value-based price for Repatha would be $2,200 to $5,000 per year, one third to one fifth the expected price resulting from the outcomes-based contract.
ICER’s value-based price estimate is now expected to fall even further, as the original calculations were based on a meta-analysis of predicted reductions in mortality using LDL cholesterol reported in various trials, which was the only measure available at the time. Results from the recently concluded FOURIER trial, however, showed no mortality benefit. Consequently, ICER announced a planned update to their original report to reflect the lower than expected benefit from Repatha, which will cause the value-based price to be an even smaller fraction of the outcomes-based price. Meanwhile, Amgen has raised the price of Repatha by 3% since launch.
Observed treatment effects in FOURIER trial vs. initial ICER estimates.
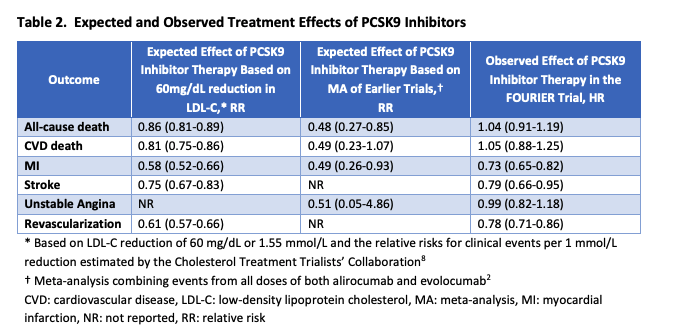
Source: Institute for Clinical and Economic Review. Evolocumab for Treatment of High Cholesterol: Clinical Effectiveness, New Evidence Update. September 11, 2017. https://icer.org/wp-content/uploads/2020/10/ICER_PCSK9_NEU_091117.pdf
What do pharmaceutical corporations say they need to engage in outcomes-based contracting?
The pharmaceutical industry has claimed that several statues and regulations need to be rolled back in order for outcomes-based contracting to be feasible. Their requests include exemptions from
- Medicaid Best Price
- Off-label promotion regulation
- Anti-kickback statute
Are these statutes and regulations really such a barrier?
Medicaid Best Price
Outcomes-based contracts have been announced in the United States for Repatha, Praluent, Entresto, Actonel, and Enbrel, among others. It seems unlikely that these contracts could be in the field and violating current Medicaid Best Price rules.
The math also does not support waiving the rule. The current Best Price standard for brand drugs is a 23% discount below AMP. Refunds to plans are probably not 100% money-back guarantees for specific patients, but instead averaged into the rebate across all patients. In order to dip below the 23% discount, failures would have to be so frequent that it should raise questions as to whether the average price is correct in the first place. For example, a refund for Repatha patients who have a heart attack or stroke would only amount to around 3.5% (according to FOURIER trial results), which would not cross the Medicaid Best Price threshold.
Finally, the claim that Best Price needs to be waived so outcomes-based contracts can be executed in Medicare is incorrect. The rule does not apply to Medicare Part D, so any discounts given to a Part D plan would not affect Medicaid Best Price.
Off-label promotion
The 21st Century Cures Act and recent FDA draft guidance have added significant flexibility to promotional activities by pharmaceutical corporations. Based on existing reports, many outcomes-based contracts already incorporate endpoints that are not included in the FDA-approved prescribing information.
Anti-kickback statute
It is hard to gauge the relative impact of the anti-kickback statue. Because the promise of outcomes-based contracts resulting in value-based prices is uncertain, it would be safer for OIG to provide protections for specific contracts, rather than take action on the statute as a whole.
How feasible are outcomes-based contracts in practice?
The experience so far casts doubt on the practical feasibility of such contracts. In fact, most of the barriers to their use are logistical; in a survey conducted with various healthcare stakeholders, only one of the three aforementioned barriers was listed (Medicaid Best Price). These other practical concerns, outlined by Garrison et al., will continue to limit the use of outcomes-based contracts, even if the requested policy concessions are granted.
What’s the potential for outcomes-based contracting?
Italy’s healthcare system has fully embraced outcomes-based contracts, which has only led to aggregate savings of 3.3% of their total drug spending from 2006-2012.
Key References:
For value-based pricing:
For outcomes-based contracting:

