R&D Costs Do Not Explain Elevated U.S. Drug Prices
Drug prices in other high income countries for the 20 top selling drugs globally make up less than half of U.S. net drug prices.
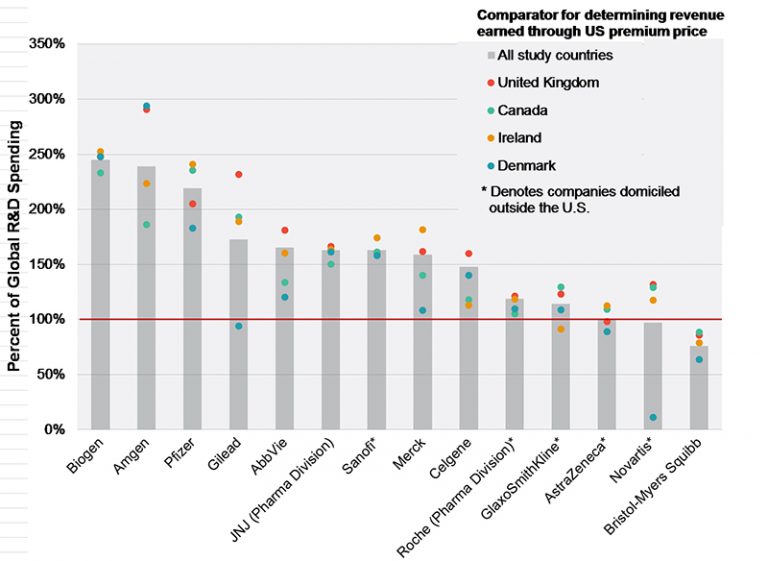
That pharmaceutical companies charge much more for their drugs in the United States than they do in other Western countries has contributed to public and political distrust of their pricing practices.
When these higher US prices (which are sometimes cited as being two to five times the prices in Europe) are challenged, the pharmaceutical industry often explains that the higher prices they charge in the US provide them with the funds they need to conduct their high-risk research.
This claim—that premiums earned from charging US patients and taxpayers more for medications than other Western countries funds companies’ research—is empirically testable. We used pharmaceutical companies' reported R&D expenses in public filings, and numerous other sources report a mix of information on their drugs’ prices and sales volumes in the US and Canada, Denmark, Ireland, and the United Kingdom. These data allowed us to quantify both the premium companies earn and the amount they spend on research. We then assessed the relation between the two.
The results show, on average, list prices in the other developed countries are only 43% of U.S. net drug prices. To put that in perspective, in 2015, the U.S. net prices were $112B higher than the other countries’ list prices, while the companies spent only $77B of that amount on global R&D.
Read the full article on the Health Affairs Blog.

Pricing Tutorials
Learn about the physical and financial flows of the supply chain using our pricing tutorials.
Use the Tool
