Academic Literature
Value-Based Pricing for Drugs: Theme and Variations
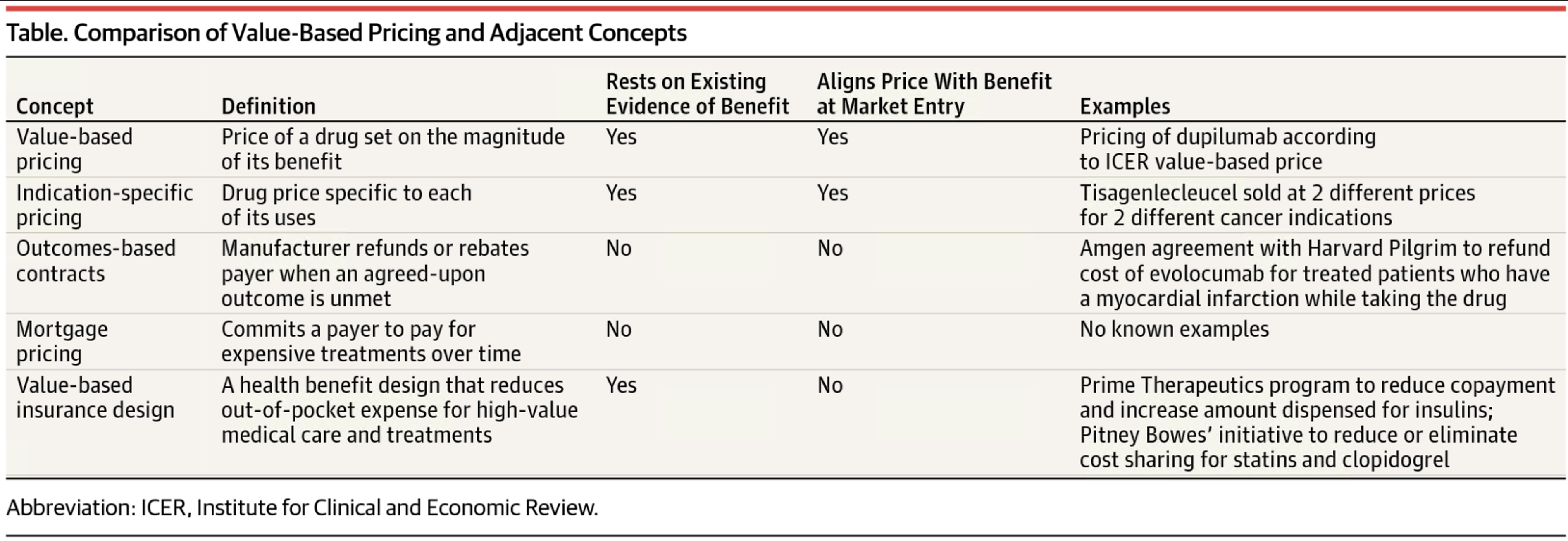
A taxonomy of the features that make a payment model truly value-based.
Originally published
on 05/02/2018
in
JAMA Viewpoint
The term "value-based pricing' has been adopted by pharmaceutical companies, payers and policy makers to refer to a range of payment models that break with convention.
However, their potential for aligning a drug's price with its value varies substantially. Anna Kaltenboeck and Dr. Bach offer a taxonomy of the features that make a payment model truly value-based.
Read the full paper here.

Share

